Is Hcl Ionic or Covalent
Does HCl have both ionic and covalent bonds. In brief hydrogen chloride is a covalent compound based on the electronegativity difference between two atoms of the HCl molecule.

Is Hcl Ionic Or Covalent Molecular Youtube
Covalent bonding are found in non-ionic.

. If the electronegativity is less than 15 then a covalent bond is present. For instance hydrogen chloride HCl is a gas in which the. In its normal gaseous state it exists as a.
It is a polar covalent compound in gaseous form while in an aqueous solution it. A case of the Ionic bond are found in the bonding of sodium. HCl and CCl4 are covalent compounds.
The Debate Over Is Hcl Ionic or Covalent. HCl is a covalent compound not an ionic compound because it is formed by sharing of one electron each by Hydrogen and Chlorine thus forming a single covalent bond. HCl Hydrogen Chloride is a covalent compound and forms a covalent bond.
Conversely if the electronegativity difference is more than 2 it is an ionic bond. Answer HCl hydrogen chloride is a polar covalent compound. Its boiling point is 8505.
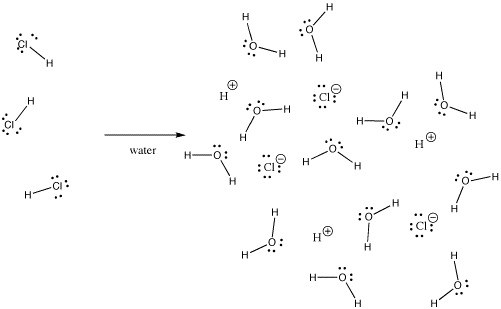
It is ionic in aqueous solution ie hydrochloric acid. To tell if HCl Hydrogen chloride is ionic or covalent also called molecular we look at the Periodic Table that and see that H is non-metal and Cl is a non-metal. H-Cl is a polar covalent molecule to a first approximation.
HCl is a binary compound of two non-metals. Case in point hydrogen chloride HCl is a gas in which the hydrogen and chlorine are covalently. It produces H and Cl- ions only in aquous solution wherein water acts as a base.
HCl is called covalent because both hydrogen and chlorine atoms share one electron with each other to. Chemical bond A chemical bond is a lasting attraction between atoms ions or. The true number of discrete water molecules within the crystal structure might be lesser.
Hcl hydrochloric acid is a covalent bond What is chemical bond ionic bond covalent bond. It confirms that the H-Cl bond in the hydrogen chloride is a polar covalent bond not an ionic bond. Many bonds can be covalent in one situation and ionic in another.
Covalent bond HCl also known as hydrochloric acid has a covalent bond. Covalent bonding are found in non-ionic substances. HCl Hydrogen Chloride is a covalent compound and forms a covalent bond.
It is covalent in gaseous state ie hydrogen chloride. Numerous bonds can be covalent in one circumstance and ionic in another. Is HCl an ionic or covalent bond.
It confirms the nature of the H-Cl bond in the HCl molecule as a polar covalent bond. HCl is both ionic and covalent. However this covalent compound has some ionic character which is calculated to be 17.
Hydrochloric acid is a simple diatomic acid whose nature of bond depends on its environment. The hydrogen H atom shares an electron with the chlorine Cl to form the bond. The hydrogen H atom shares an electron with the chlorine Cl to form the.
However if water is added to hydrogen chloride it forms hydrochloric acid which is an ionic. Hence they cannot be ionic. It is another criterion for distinguishing between Ionic and covalent.
What type of bond is HCl. HCl also known as hydrochloric acid has a covalent bond. However if water is added to hydrogen chloride it forms hydrochloric acid which is an ionic.
In this state it is not acidic at all. Satisfy their respective valence shells and hence they form. When atoms combine to form molecules the bonds between them may be of two kinds ionic or covalent.
And of course we know that H-Cl is molecular because it is supplied in the lab as a gas. Hydrogen chloride or HCl is a covalent polar covalent compound because when one nonmetal combines with another nonmetal it usually forms a covalent compound. Is HCl polar or ionic.
In aqueous it gives H.

Is Hcl Ionic Or Covalent Molecular Youtube

Why Is Hydrogen Chloride Hcl A Covalent Compound Why Can T It Be An Ionic Compound Quora
Hcl Is A Covalent Compound Why Doesn T Hydrogen Give Its One Electron To Chlorine The Chlorine Will Become Stable By Completing Its Octet Quora

Is Hcl Ionic Or Covalent Techiescientist

Comments
Post a Comment